Acetone Polar or Nonpolar Solvent
Hydrophobicity is most readily understood from the perspective of the contact angle of water on the surface of materials. An example of a polar solvent is water while ethanol is more nonpolar.
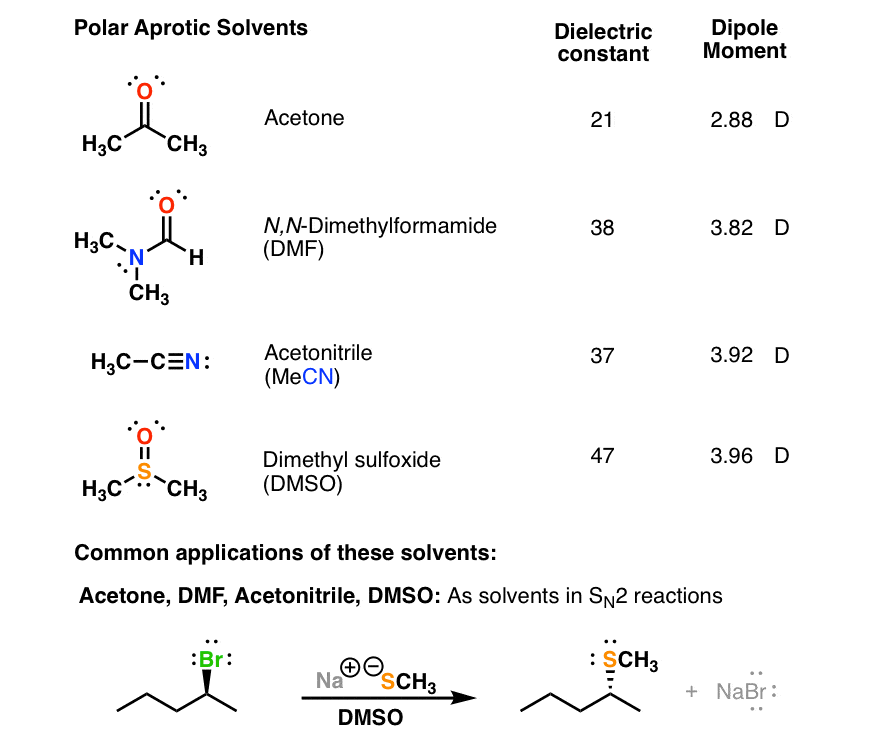
Polar Protic Polar Aprotic Nonpolar All About Solvents
Phase is nonpolar then nonpolar pigments move further up the paper.

. When a small drop of water to neglect gravitational effects 16 is placed on a material a boundary line occurs between the water the substrate and air Fig. This is because Like dissolves like that is pigments with a chemical composition similar to the composition of the solvent will dissolve better in that solvent and therefore move further up the paper. 131The angle Θ that a water droplet takes on the surface can be determined from the energy balance.

Is C3h6o Acetone Polar Or Non Polar Youtube
Polar Vs Nonpolar Solvents Identifications And Examples Psiberg
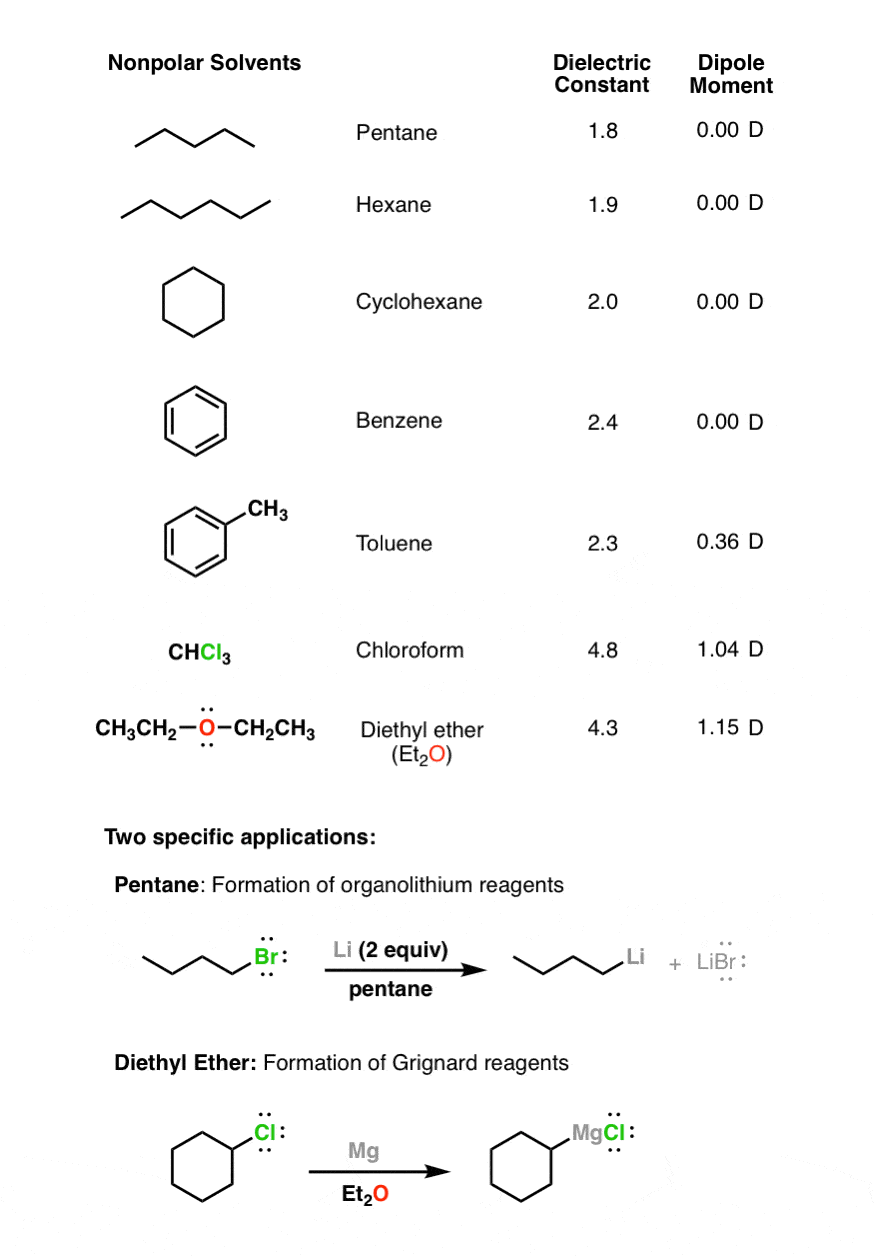
Polar Protic Polar Aprotic Nonpolar All About Solvents

Why Is Acetone A Good Solvent Properties Explanation Video Lesson Transcript Study Com
No comments for "Acetone Polar or Nonpolar Solvent"
Post a Comment